There has been a lot of influenza A in my community. One of my 79 year old patients was just sent home after a harrowing week in the hospital with influenza A, including 3 nights in the ICU. She was vaccinated but has some medical issues like most people her age. So I’ve been thinking about whether prescribing Tamiflu makes sense for people, for prevention of flu spread in households, and what happens if there’s an H5N1 pandemic. What does recent evidence say about these questions, and what does the CDC recommend before more lights go out?
In this post I’m going to review the preferred antiviral treatments of influenza, namely Tamiflu which most people have heard of, and a newcomer called Xofluza. Tamiflu is cheap, available right now, and stockpiled to some degree. Most people have not heard of Xofluza. It is more expensive, hard to get from pharmacies in a timely manner, and not stockpiled much. I’ll then share the CDC recommendations for treatment of H5N1 avian influenza right now, and how this might unfold if our pre-pandemic situation evolves. And finally I’ll end with a shout out to flu vaccines, and compare how they stack up against these antivirals.
The two main antivirals
In primary care (and in the hospital) we mostly use Tamiflu (generic name oseltamivir) and sometimes Xofluza (generic name baloxavir) when treating flu.
Tamiflu works by inhibiting viral neuraminidase, preventing newly formed virus particles from detaching from infected cells, while Xofluza inhibits the viral cap-dependent endonuclease, blocking viral mRNA synthesis and preventing viral replication.
Here’s what UpToDate has to say about the evidence and efficacy of using Tamiflu or Xofluza for treating influenza (it’s behind a paywall unless you subscribe like me):
Tamiflu/oseltamivir
Preferred first-line treatment for both outpatient and hospitalized patients
Approved by FDA in 1999. Taken twice daily for 5 days.
Reduces symptom duration by approximately 16-24 hours compared to placebo
In high-risk outpatients, treatment within 48 hours showed:
Shorter median time to symptom resolution (97.5 vs 122.7 hours)
Reduced lower respiratory complications like pneumonia requiring antibiotics (4.9% vs 8.7% absolute risk reduction, which is a 44% relative risk reduction)
Modest reduction in hospitalization rate (0.6% vs 1.7% absolute rates)
Main side effects include nausea and vomiting (5-10% report this)
Resistance remains rare ( >95% of current isolates are susceptible)
Xofluza/baloxavir:
Approved in 2018 for uncomplicated influenza in adults within 48 hours of symptoms, and more recently for post-exposure prevention of influenza.
A single oral dose reduces symptom duration by approximately 29 hours vs placebo
In the CAPSTONE-1 trial, median time to symptom resolution was 53.7 hours vs 80.2 hours with placebo
Similar efficacy to oseltamivir but with fewer gastrointestinal side effects
Not recommended for immunocompromised patients due to higher resistance risk (10% developed resistance in trials)
Main side effect is diarrhea (1.8% vs 1.3% with placebo)
To brush up on your ninja biostatistics skills regarding relative and absolute risks, please refer to this ninja guide.
Update on efficacy of these antivirals
We have a tendency to give higher credence to the most recent study we are reading, and to discount the older ones. And so when a new meta-analysis just published in JAMA Internal Medicine this month came out, it really rained on the Tamiflu parade. But this is just one study to consider, with the upsides and downsides of large meta-analyses baked in. A large meta-analysis has the upside of providing a more precise estimate of an effect size by combining data from many studies, increasing the overall statistical power, and potentially resolving conflicting results from individual studies. However, downsides include the potential for publication bias, heterogeneity between studies (different methodologies), and the complexity of analyzing and interpreting large datasets, which can lead to misleading conclusions if not carefully conducted.
But here it is to consider.
Based on this systematic review and meta-analysis of 73 trials with 34,332 participants:
Tamiflu/oseltamivir
Had little to no effect on hospitalization risk (reduction of 0.4% absolute risk)
Reduces symptom duration by 0.75 days compared to placebo, considered not clinically important
Increases treatment-related adverse events (like nausea/GI effects) by 2.8% compared to placebo
No effect on mortality in either low or high-risk patients
No meaningful impact on ICU admission rates
Xofluza/baloxavir
May reduce hospitalization risk in high-risk patients by 1.6% absolute rate compared to standard care/placebo, though evidence certainty is low
Reduced symptom duration by 1.02 days compared to placebo (moderate certainty)
Did not increase treatment-related adverse events (reduced by 3.2% vs placebo)
Main concern is emergence of resistance to baloxavir in approximately 10% of treated patients
No effect on mortality in either low or high-risk patients
This new study concludes that baloxavir may be superior for high-risk patients with non-severe influenza, while oseltamivir shows limited benefits and increases adverse events. And yet one study does not negate all others before it, so Tamiflu still has a place at the head of the table. Read on.
Availability
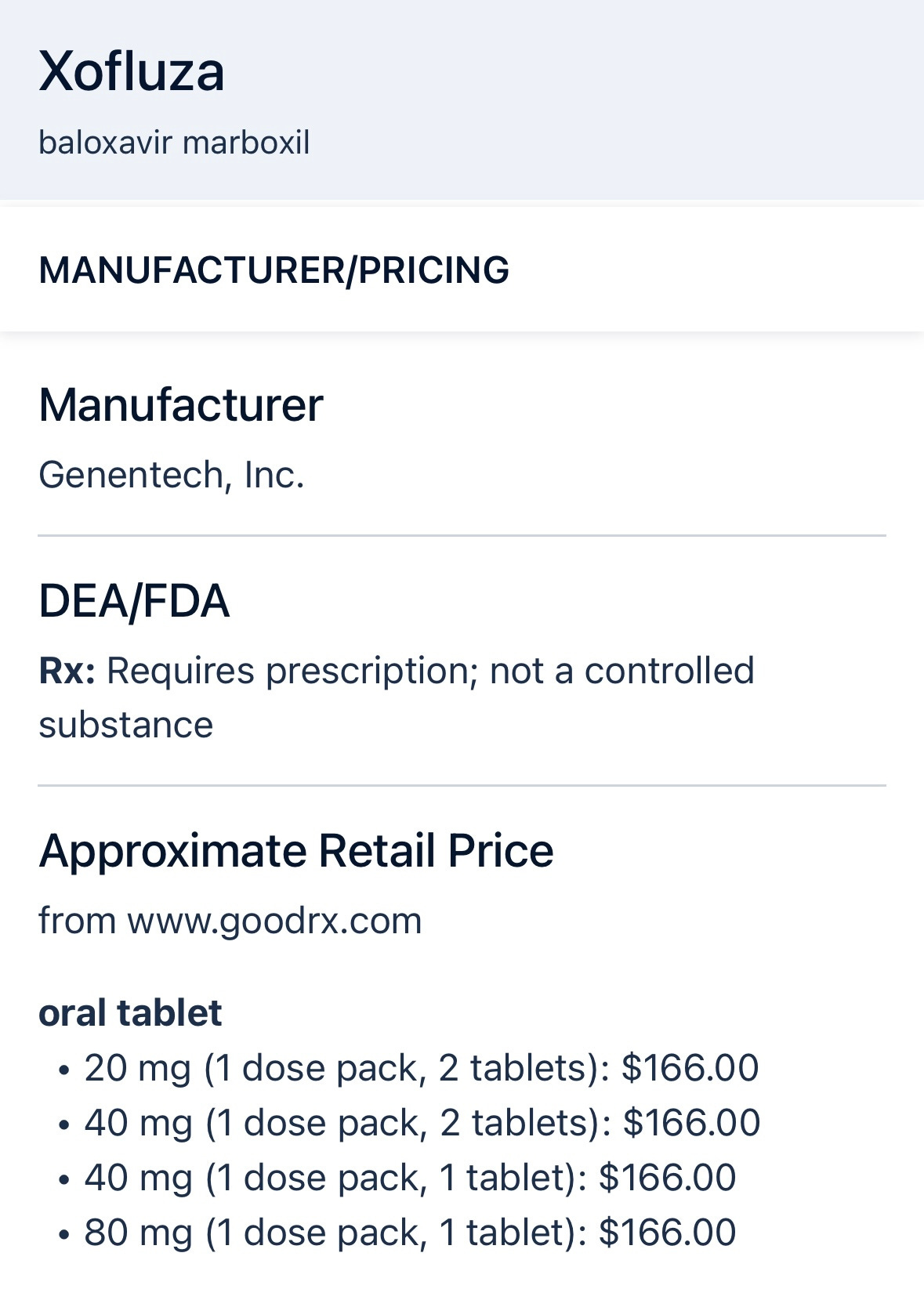
Seems like we should be prescribing more Xofluza, but this is logistically difficult for several reasons. First, it’s more expensive than Tamiflu ($166 versus $15 or so). Insurance companies love to deny my prescriptions, and time is of the essence, so their refusals waste critical time. Secondly, because of this ubiquitous problem, most pharmacies do not stock Xofluza in my experience. For example, while writing this post and treating some people for influenza this week, I called a local pharmacist. They confirmed “no Xofluza,” but if I ordered it they could get it in stock within a day or two. That misses the critical 48 hour window from the start of symptoms.
Here’s a communication I got just yesterday after trying to prescribe Xofluza:
State plan only covers this drug when patient meets one of these options: (1) they have tried other products plan covers (preferred products) and these did not work well. OR (2) doctor documents and provides a medical reason as to why the preferred products can't be taken.
Preventing influenza transmission within households
If someone is sick with the flu, what about starting their household contacts on Tamiflu or Xofluza? I get this call often, and I usually oblige. What do the guidelines state about this practice?
For post-exposure prophylaxis, oseltamivir and other antivirals are recommended only in limited situations:
for people at very high risk of complications (like immunocompromised)
for unvaccinated household contacts when another high-risk individual(s) also live there.
treatment must start within 48 hours of exposure
The evidence shows oseltamivir is quite effective - it reduces the risk of symptomatic influenza by 60% according to one meta-analysis, while another systematic review found 81% efficacy. However, there's no data on whether it prevents serious complications.
Early treatment of symptoms in family members of known influenza contacts is considered a reasonable alternative to prophylaxis.
Tamiflu versus Xofluza, head to head in the house
Which choice works better to reduce spread?
Based on a 2024 post-hoc analysis of the BLOCKSTONE trial, Xofluza appeared more effective than Tamiflu at preventing household transmission of influenza.
The adjusted secondary attack rate (family members getting sick) was 10.8% in household contacts of Xofluza-treated index cases versus 18.5% for Tamiflu-treated cases, representing a 41.8% relative risk reduction.
The greater effectiveness of Xofluza in this study may be due to its more rapid reduction in viral load and its simpler single-dose regimen compared to Tamiflu’s twice-daily dosing for 5 days.
H5N1 speculation
Here are some treatment guidelines and estimates of efficacy from prior avian flu types. As we increasingly tilt towards a mutation that will allow H5N1 to pass between humans, it’s important to consider these antivirals. We have no idea how well they would actually work in a pandemic, since the future potential mutated virus by definition does not yet exist. However, according to current CDC guidelines…
Tamiflu/oseltamivir is the preferred treatment for both hospitalized and outpatient cases of avian influenza. It’s what they have given dairy workers and other sporadic cases so far. The standard adult regimen is 75 mg twice daily for five days (same as what we use for regular flu), though hospitalized patients with severe illness may require up to 10 days of treatment. The evidence for oseltamivir's effectiveness comes from substantial registry analyses and observational studies.
A 2010 registry analysis of over 300 H5N1 patients demonstrated that oseltamivir reduced mortality significantly by 50%.
Timing of treatment is crucial: A 2018 study of 160 hospitalized H7N9 (another avian influenza virus) patients showed mortality rates of:
15% when treated within 2 days
23% when treatment started between 2-5 days
37% when treated after 5 days.
A 2012 registry study of H5N1 cases found early treatment resulted in dramatically lower case-fatality rates (18% vs 63% for delayed treatment). In children with H5N1, each day of delayed treatment increased death risk (odds ratio 1.75).
Xofluza/baloxavir, while FDA-approved for seasonal influenza, is not preferred for hospitalized avian influenza patients due to limited efficacy data. The studies just aren’t out there yet. But it can be used as an alternative for outpatients with mild-to-moderate illness who present within 48 hours of symptoms (40 mg for patients 40-80 kg, 80 mg for those over 80 kg, as a single dose).
While laboratory studies show baloxavir has activity against H7N9 in animals and cell models, clinical experience in human avian influenza cases remains limited. It may serve as an alternative therapy in cases of oseltamivir resistance.
Here are some additional thoughts and considerations, expanded from the CDC guidance on treating avian influenza.
Treatment should be initiated immediately in suspected cases, even before test results return and regardless of time since symptom onset. This includes patients with mild symptoms like conjunctivitis due to progression risk.
Combination therapy with oseltamivir and baloxavir is recommended for immunocompromised patients to prevent resistance, though clinical trials show limited benefit.
Standard oseltamivir dosing (75mg twice daily) is adequate even in critically ill patients, with no proven benefit to higher doses.
Let’s really hope this avian influenza stuff does not become a human pandemic. Let’s hope we have excellent public health leadership if it does. Let’s hope for a deep appreciation for science and a healthy respect for scientists among our elected leaders.
🤔
Stockpiles
I’m not privy to how much Tamiflu is stockpiled, but federal and state governments should be actively making sure we have lots. Xofluza is not being stockpiled to the degree that Tamiflu is. Most states have none. See cost discussion above.
Good old flu shots for regular flu
Hopefully you got yours already. It’s not too late to get one if you haven’t. Still lots of influenza A out there, and influenza B usually comes next.
The effectiveness of the influenza vaccine varies by age group and the outcome being measured, such as transmission, hospitalization, or mortality.
In children (6 months to 5 years), vaccine effectiveness (VE) is often the highest, exceeding 50% in many cases, providing strong protection against all influenza subtypes.
Adults aged 18–49 years typically experience moderate VE, ranging from 40% to 60%, with better protection against influenza B and A(H1N1) compared to A(H3N2).
Older adults (65+ years), however, tend to have lower VE due to weaker immune responses. For instance, VE against A(H3N2) can be as low as 14%, although high-dose vaccines slightly improve protection.
In terms of specific outcomes, influenza vaccines reduce household transmission by approximately 21%, with higher effectiveness against influenza B (up to 56%) than influenza A. Vaccination reduces hospitalization rates by 35% to 61%, depending on the season and strain match, and lowers mortality risk by about 18%, particularly benefiting high-risk groups. Overall, vaccine effectiveness is highest in children and during seasons when the vaccine strains closely match circulating viruses. Tailored approaches, such as high-dose vaccines for older adults, help improve protection in vulnerable populations.
In a previous post last month I shared speculation about whether the regular flu shot might help protect against severe H5N1 disease if that takes off. The answer is maybe. We don’t know, but there are a couple indirect studies to consider therein.
Conclusion
While both Tamiflu and Xofluza offer benefits in treating influenza, recent evidence suggests their impact may be more modest than previously thought. Xofluza appears to have some advantages over Tamiflu, including better efficacy in preventing household transmission and fewer side effects, but its higher cost and limited availability pose practical challenges for prescribing.
For avian influenza H5N1, Tamiflu remains the preferred treatment based on observational data showing significant mortality reduction, particularly when administered early.
The seasonal flu vaccine, despite varying effectiveness across age groups and strains, continues to be our best preventive tool, reducing transmission, hospitalization, and mortality rates. As we face the ongoing threat of both seasonal and potentially pandemic influenza strains, maintaining adequate antiviral stockpiles and ensuring widespread vaccination coverage remain crucial public health priorities, while being prepared to adapt treatment approaches based on emerging evidence.
Hang in there.
They can’t stop the clock on this flu season.
Spring is coming.






Such an impressive deep dive, Doc. I didn’t know there was a second flu drug. It does seem like shaving a day or less of the flu may not be worth the side effects. And the chase to find the X one likely misses the critical window for starting it, as you’ve pointed out.
Here’s a chuckle (or maybe not, considering current epidemiological events): the only flu shot I ever got was about a month before I got COVID (I’m not drawing a correlation there, it was just the timing). I got it in my upper left arm, where I have a giant tattoo of a raven reading a book while standing on a stack of books. I joked at the time that it was my bird flu shot 🙃. (I’ll see myself out now 🤦🏼♀️).
Thank you for this pertinent information Dr. McCormick. As a physiologist, I recommend vaccines to all in my family. I was so grateful when they finally allowed adults to receive flu shots. When I was a teacher I caught one or another flu virus every year single year, sometimes twice. Ever since flu shots have become available I haven't missed a year and haven't had flu. I also keep COVID shots current. My children got every vaccine available so they avoided all childhood diseases for which vaccines were available.
When I was a child (1933 to 1945) I caught every damned childhood disease that came around. I wish we could develop vaccines against cancer and I'm sure those will come in the future. Cancer killed 3 of my 4 children - the other child died from mental health complications.
Now with mRNA vaccines, I'm very hopeful that we'll conquer more diseases.