RSV vaccine - a primary care update on serious risks and benefits
Old data, plus real world stuff
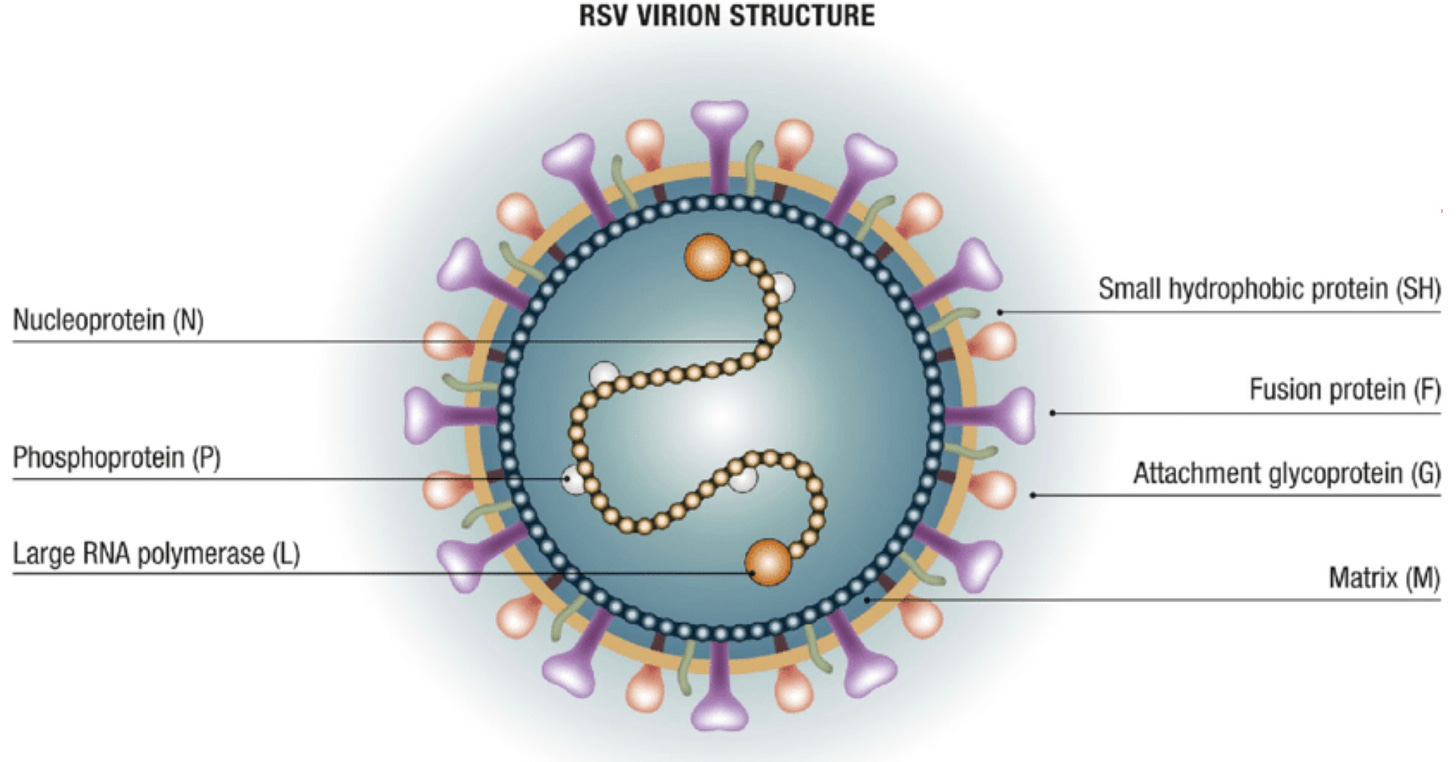
This fall marks the second year that we’ve had Respiratory Syncytial Virus (RSV) vaccines to offer older adults. Chances are you have received one, are eligible to receive one, or have older family/friends who qualify but are wondering what to do. I’ve been fielding lots of questions about the RSV vaccines from patients over the past several weeks… an…
Keep reading with a 7-day free trial
Subscribe to Examined to keep reading this post and get 7 days of free access to the full post archives.