Does Prevagen Really Work for Memory Loss?
Is apoaequorin a great fix? A difficult word to pronounce? Or a jelly fish protein that's just another profitable disappointment?
One of the most frustrating (and often terrifying) experiences as we get older can be a loss of cognitive powers. It’s one of the most frequent concerns I hear from people. Often the next question is about supplements like Prevagen. The promise of ways not to become more forgetful with age is seductive. And we would spend almost anything to help ourselves and our loved ones avoid the merciless decline of actual diseases like Alzheimer’s.
Some degree of forgetfulness is only natural as the brain accumulates plaques, tangles, and rust on an unfathomable neural network composed of 100 trillion connections. There is just no way we can “picture” that sort of complex structure. The entire universe has just 200 billion galaxies. Our Milky Way has only 100 thousand million stars. And so the question of how to improve a human brain’s 100 trillion connections is by nature a very complex one.
Prevagen, the world’s “#1 Pharmacist Recommended Memory Support Brand in 2022,” claims they have partially cracked the code. No wonder I am frequently asked: Does Prevagen work?
It is made by a company called Quincy Bioscience. The only product I can find on their website is Prevagen, and a “NeuroShake” protein drink. Quincy is not a biotech start up with an exciting pipeline of medicines. They offer 60 pills of Prevagen Extra Strength that will run you about $110 on the company website. If you take Prevagen once a day as recommended, that’s $660 for a year. That sort of money is not insignificant. It is equivalent to a 14 year subscription to this letter, or one tankful of gasoline.
So let’s go through the claims made right there on the Prevagen company website:
Clinically shown to help with mild memory loss associated with aging.*
In a computer-assessed, double-blinded, placebo-controlled clinical study, Prevagen improved certain aspects of cognitive function over a 90 day period in subgroups of individuals who were cognitively normal or mildly impaired.*
The *asterisks* at the end of each claim are actually disclaimers. Scrolling to the bottom of the page, if your eyes can still read the smaller font, you will find:
* These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
Evaluation by the FDA is important. In fact, it is a necessary precondition for the actual pharmaceuticals that doctors can prescribe. Prescription drugs have been evaluated by the FDA for safety, manufacturing quality control, and proven efficacy. Most supplements have not. Why this is allowed to happen is the subject for another post, but many other skeptics have followed the money. At $40 billion dollars a year, the revenue from the supplements industry is out of control. FDA recently announced they are working to improve the system
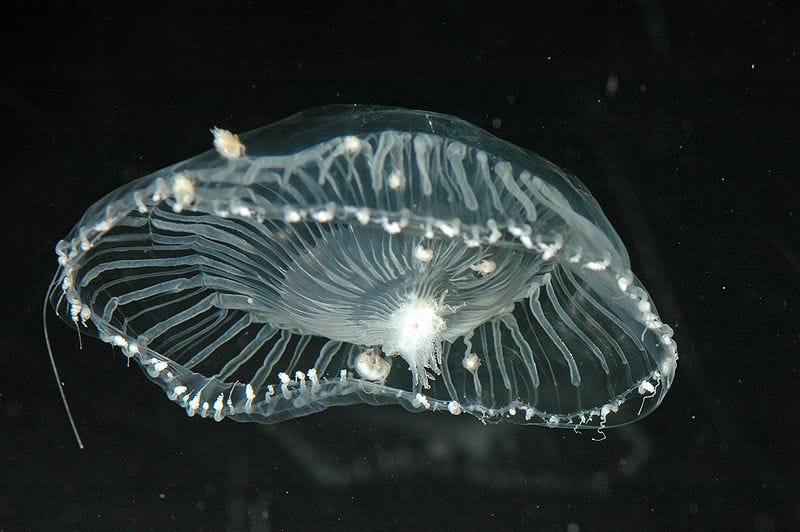
Back to Prevagen. The prime ingredient is called apoaequorin. It is a protein found in glow-in-the-dark jellyfish. Here’s the reasoning as to how jellyfish might be relevant to human cognition: apoaequorin is a calcium binding protein. In the human brain, regulation of calcium is important. An imbalance of calcium metabolism in neurons has been associated with cellular damage and disruption of connections. Therefore, apoaequorin’s ability to bind calcium might help.
Experts have found that the apoaequorin in Prevagen is probably digested and broken down in the stomach like other proteins, long before it even has a chance of reaching the brain. You could almost stop reading here, but let’s keep going.
So what about the “computer-assessed, double-blinded, placebo-controlled clinical study.” That sounds pretty legitimate. But if you dig deeper into the study presented by Quincy Bioscience as proof that their supplement works, you find that the data is pretty weak. There were 30 measures of memory assessed by something called the Madison Memory Study. Overall no statistically significant benefit was found between Prevagen and placebo. But if the 30 or so unique measures are considered individually, there emerged a possible improvement in about 3 of those 30 challenges. The Washington Post stated this about the clinical trial Prevagen leverages its scientific legitimacy upon:
But, according to the FTC and the New York attorney general, the trial involved 218 subjects taking either 10 milligrams of Prevagen or a placebo and “failed to show a statistically significant improvement in the treatment group over the placebo group on any of the nine computerized cognitive tasks.”
The complaint alleges that after the Madison Memory Study failed to “find a treatment effect for the sample as a whole,” Quincy’s researchers broke down the data in more than 30 different ways.
“Given the sheer number of comparisons run and the fact that they were post hoc, the few positive findings on isolated tasks for small subgroups of the study population do not provide reliable evidence of a treatment effect,” the lawsuit said.
I checked out the actual study results posted on the Prevagen website. The clinical trial was not published in any respectable, peer-reviewed medical journal that I could find. The principal investigator is named Kenneth C. Lerner, and from his public LinkedIn profile he has BS, MS, and MBA degrees. He is listed as “Business Development and Intellectual Property Manager” for Quincy Bioscience. Obviously the makers of Prevagen funded and ran this study, with their principal investigator also a principal executive.
When the prospective part of the trial disappointed, the company then hired a team of researchers and number crunchers to go back (post-hoc) and mine the data for anything that approached clinical significance. They were able to craft a couple comparisons that showed mildly favorable results. According to a legal brief filed by AARP and TruthInAdvertising.Org, the results “are sliced and diced in multiple overlapping ways… decreasing their sample sizes and simultaneously increasing the chances of getting a false positive.” And even the cherry picked tests are not the best ones used to assess executive functioning and memory, and have not been validated like the gold standard tests in this field.
Despite lawsuits, a class action settlement, and an ongoing FTC investigation, Prevagen is still going strong. Ask you doctor about… and I am often asked about it.
Beyond the dubious study results noted above, Prevagen makers also highlight testimonials through a series of compelling stories and videos. I find some of the videos cringe-worthy: people throwing horseshoes together, exploring lush rose gardens, analyzing computer screens filled with mysterious data. It’s the same kind of uneasiness I feel watching Viagra or other pharmaceutical commercials on TV. Slick and inviting. And then it feels creepy prescribing otherwise good medications.
If you would like to continue reading some excellent journalism on the subject of Prevagen, this article in Wired magazine is quite definitive. It also digs into the personal history of the inventor of Prevagen, named Mark Underwood (paraphrased):
The idea that a protein found in jellyfish could improve the human brain occurred to Underwood serendipitously, as he tells it. In his self-published book The Brain Health Guide, he describes how, at the library one evening in the 1990s, he came across an article about a swimmer who developed neurological symptoms after a jellyfish sting. It was then that his inspiration struck, he writes: “Could what was in the jellyfish help to support humans, specifically our brains, in the same way as the jellyfish?”
His parents remember it differently.
…Underwood’s ambition, noted under his senior photo, was different: “Make a totally obnoxious amount of money at an early age and spend the rest of my life spending it.”
(His mother) has multiple sclerosis, a chronic disease that can attack the central nervous system. It turned her into a “medical junkie,” she says… she was attracted to the way jellyfish seemed to move so easily. That’s what led her to wonder if the marine animal might hold the key to a medical breakthrough. Diane says she shared her inspiration with her son while he was studying at the University of Wisconsin-Milwaukee, and that he took to the idea “like a dog with a bone.”
~
In addition to Prevagen, I’ve also fielded questions about Neuriva recently. It’s probably not worth a separate post, so instead I’ll point you towards a helpful article from Psychology Today. Neuriva is unlikely to be worth your money either.
~
So what should you do?
The makers of Prevagen show this graph on their website, implying that your local pharmacist (and maybe family doc) are on board with Prevagen to support memory. They further summarize: “Of pharmacists who recommend a memory support brand, Prevagen was far and away the number one recommended product. The survey shows that millions of recommendations are made each year to customers in the area of non-prescription memory support.”
Note that the *asterisk* makes another appearance here. And I wonder what percentage of pharmacists actually recommend any memory support brand…
Stay tuned for a separate post on how we can best sustain our memory, cognitive function, and brain powers. Most of the advice will be low tech, intuitive, but worth learning or reviewing. But for this post about Prevagen, I'll keep it brief with a bit of advice from Pharmacy Today that I’m assuming most pharmacists would agree with:
Memory problems are a concern for many older adults. Pharmacists should educate patients that normal cognitive aging occurs and is not a disease. Regular physical exercise, a healthy diet, social engagement and lifelong learning, as well as the avoidance of inappropriate medications, are essential and likely possess additional health benefiits.
Although supplements such as omega-3 fatty acids have been promoted for improving memory, trial results have been mixed. No benefit was shown in a major rigorous study, even with omega-3 fatty acids. Human data on apoaequorin are limited to small, company-sponsored trials that do not meet expected scientific standards.
~
And so in conclusion, when I am asked about whether I, as a family doc, recommend Prevagen - I generally say nah.
I hope no one feels judged or embarrassed by this post. Often we feel desperate for help, and the medical community does not have all the answers or solutions. I do have a deep appreciation and compassion for how challenging it is to grow older, both from what I have attended with my patients for over 20 years, and from what I am already experiencing in my own body. Just wait they say! And we have all felt that powerlessness to slow time, or to help an aging loved one as they endure the slings and arrows. And so I’ll cobble together some evidence-based advice for the next post… and be assured (and perhaps a little disappointed) that it will not contain many solutions in a bottle.
******* ****** ******* ******* ******** ******* ******* ****** ******* ******* ******* ********** ****** ****






I’m impressed. Factual but very lovingly presented. Great article!
Excellent analysis. Thank you.